
Calculating Mr from moles and mass YouTube
A tutorial showing how to use the relative formula mass (Mr) or relative atomic mass (Ar) to calculate the mass of a substance given its moles or the number of moles in a substance.
Moles and Calculations Gidemy Class Notes
One mole of NH 3 molecules - which has a relative molecular mass (Mr) of 17 - has a mass of 17 g, and half a mole has a mass of 8.5 g. One mole of ibuprofen (C 13 H 18 O 2) has a mass of 206 g, and 0.01 moles have a mass of 2.06 g (which is still way more than is in an ibuprofen tablet). Moles allow us to compare the number of atoms or.

Mole calculations 2 Mass = Mr x mole YouTube
The molar mass of a substance is defined as the mass of 1 mol of that substance, expressed in grams per mole, and is equal to the mass of 6.022 × 10 23 atoms, molecules, or formula units of that substance. Key Takeaway. To analyze chemical transformations, it is essential to use a standardized unit of measure called the mole.
PPT Mole Calculations PowerPoint Presentation ID902714
The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). If the mass of a substance is known, the number of moles in the substance can be calculated. Converting the mass, in grams, of a substance to moles requires a conversion factor of (one mole of substance/molar mass of.

calculating molarity worksheet
Mass from Moles. Mr. Causey shows you step by step how to use molar mass to find the mass of a compound in moles. http://www.yourCHEMcoach.comLearn more and.

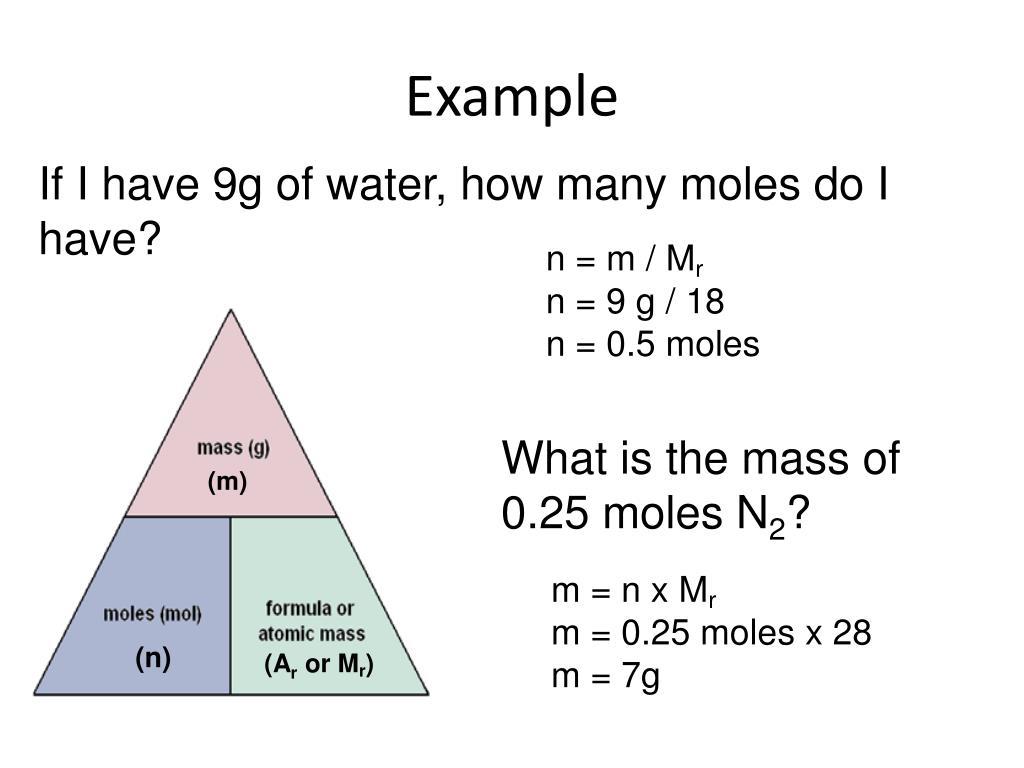
How to calculate the number of moles? The formula for the number of moles is mass divided by
The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. The representative particles can be atoms, molecules, or formula units of ionic compounds. This relationship is frequently used in the laboratory. Suppose that for a certain experiment, you need 3.00 moles of calcium chloride \(\left.

Reacting amounts calculations YouTube
The molar mass of a substance is defined as the mass of 1 mol of that substance, expressed in grams per mole, and is equal to the mass of 6.022 × 10 23 atoms, molecules, or formula units of that substance. KEY TAKEAWAY. To analyze chemical transformations, it is essential to use a standardized unit of measure called the mole.

How to Calculate Mass from Moles of a Compound Mr. Causey's Chemistry YouTube
Mass and Mole Calculations. The mole is a unit in chemistry that tells us the amount of a substance. It gives an indication of how many particles are present in a substance.. We can also work out the masses of the products using the mass = moles x Mr equation. 0.1 x 63.5 = 6.35 g of copper. 0.05 x 44 = 2.2 g of carbon dioxide. View fullsize.

IGCSE Edexcel Chemistry Help 1.19 carry out mole calculations using relative atomic mass (Ar
The value of a mole is fixed, it does not change with the substance being discussed, i.e. one mole of iron, one mole of electrons, and one methane molecules both contain 6.022 \times 10^{23} particles.This number is known as the Avogadro constant and is typically give the symbols L or N A.. For any given substance, the mass of one mole (6.022 \times 10^{23} particles) of a substance will be.
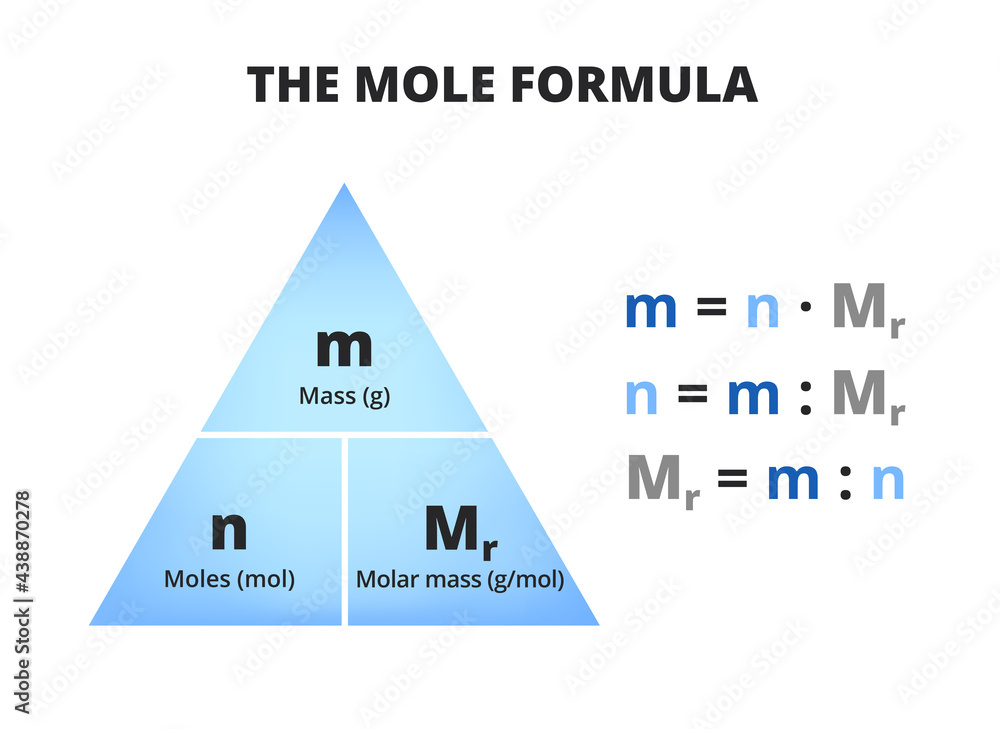
The mole formula triangle or pyramid isolated on a white background. Relationship between moles
The molar mass of a substance is defined as the mass of 1 mol of that substance, expressed in grams per mole, and is equal to the mass of 6.022 × 10 23 atoms, molecules, or formula units of that substance. Key Takeaway. To analyze chemical transformations, it is essential to use a standardized unit of measure called the mole.
PPT Moles and reacting masses PowerPoint Presentation, free download ID2568915
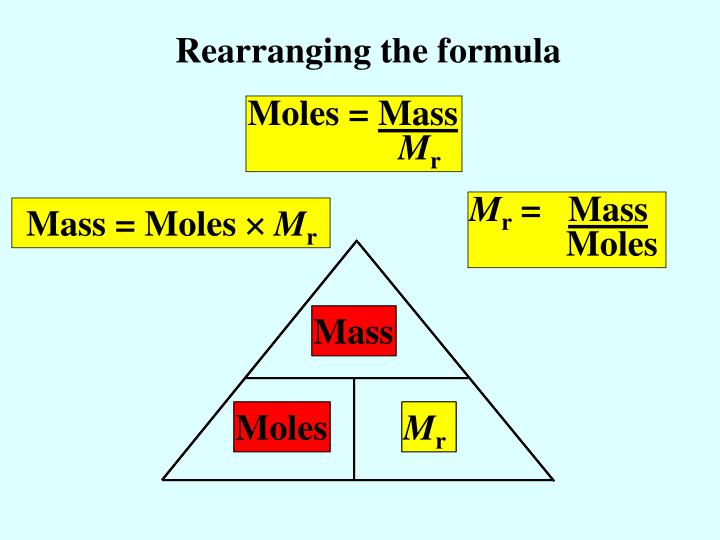
One mole is the formula mass (Mr) of any substance in grams, and it is Avogadro's number. Putting these two together, we can say that in one mole of the mass of any substance, there are 6.02 x 10 23 particles. We can use the equations to find the number of particles in any given mass of substance: Number of moles = Mass/ Mr = given number of.
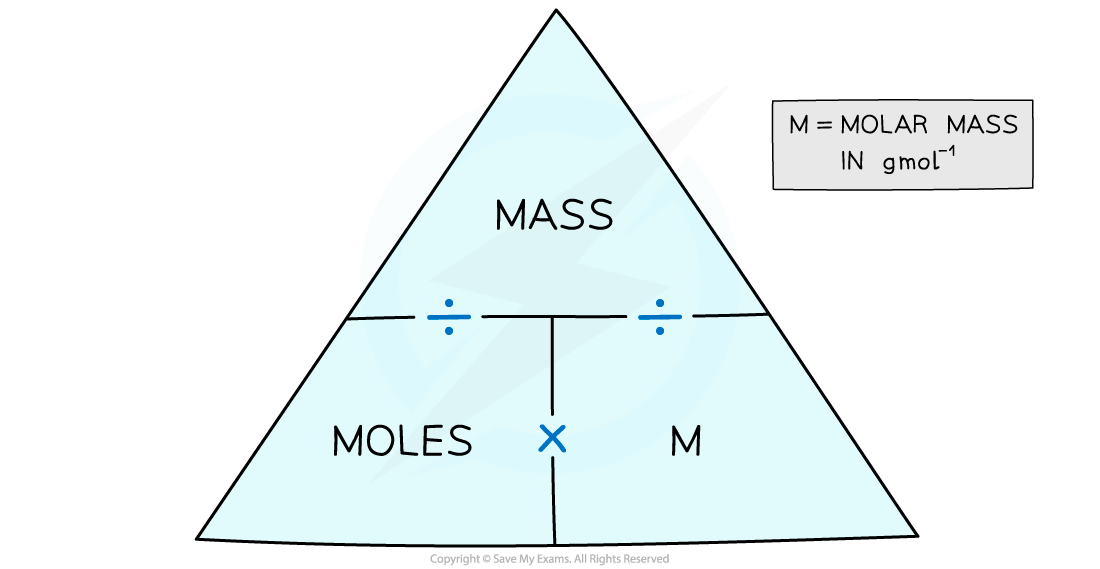
IB DP Chemistry HL复习笔记1.1.5 MolesMass Problems翰林国际教育
The molar mass of a substance is the mass of one mole close mole The amount of substance that contains the same number of particles as there are atoms in 12 g of carbon-12 (contains the Avogadro's.
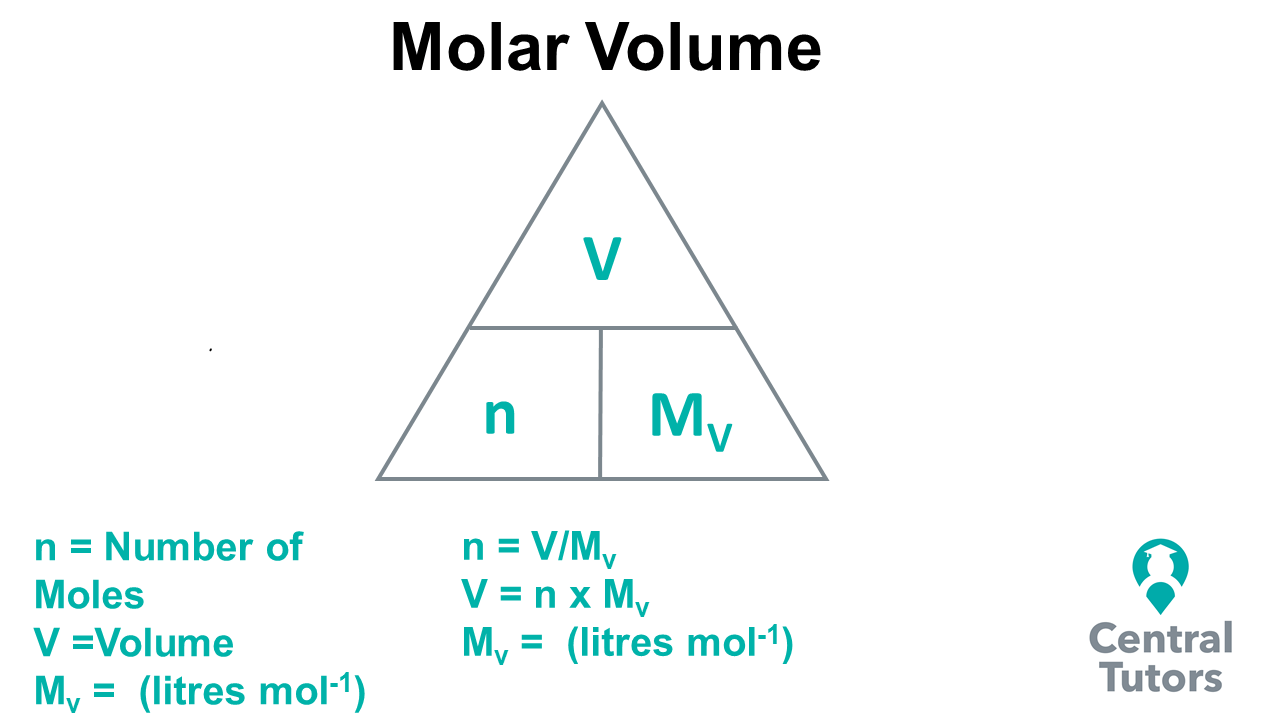
Early Mole Calculation Overview A Level Chemistry vlr.eng.br
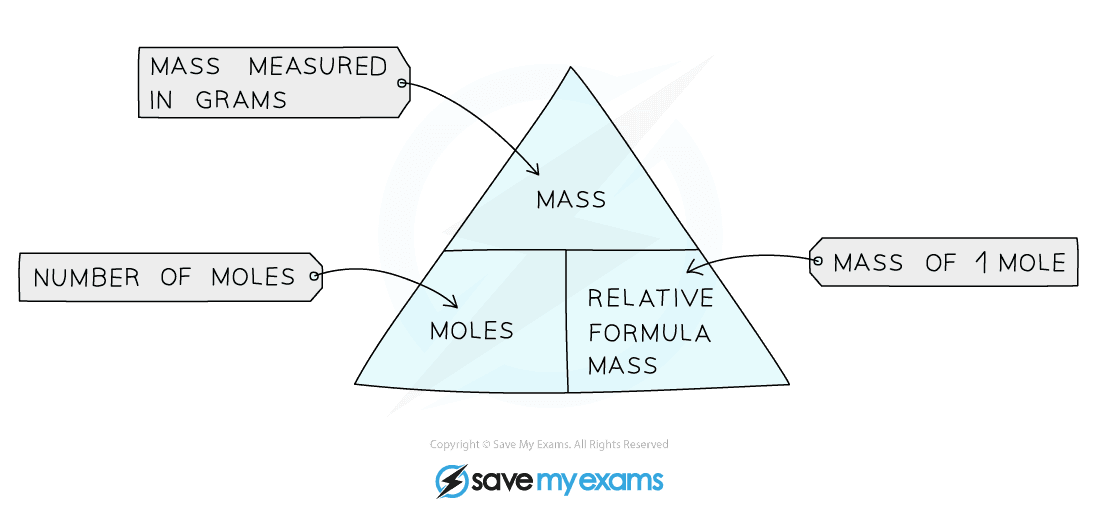
Calculating amounts in moles Key fact The amount of a given mass substance is calculated using: \ (amount=\frac {mass} {relative~atomic~or~formula~mass}\) Use Ar instead of Mr for metals.
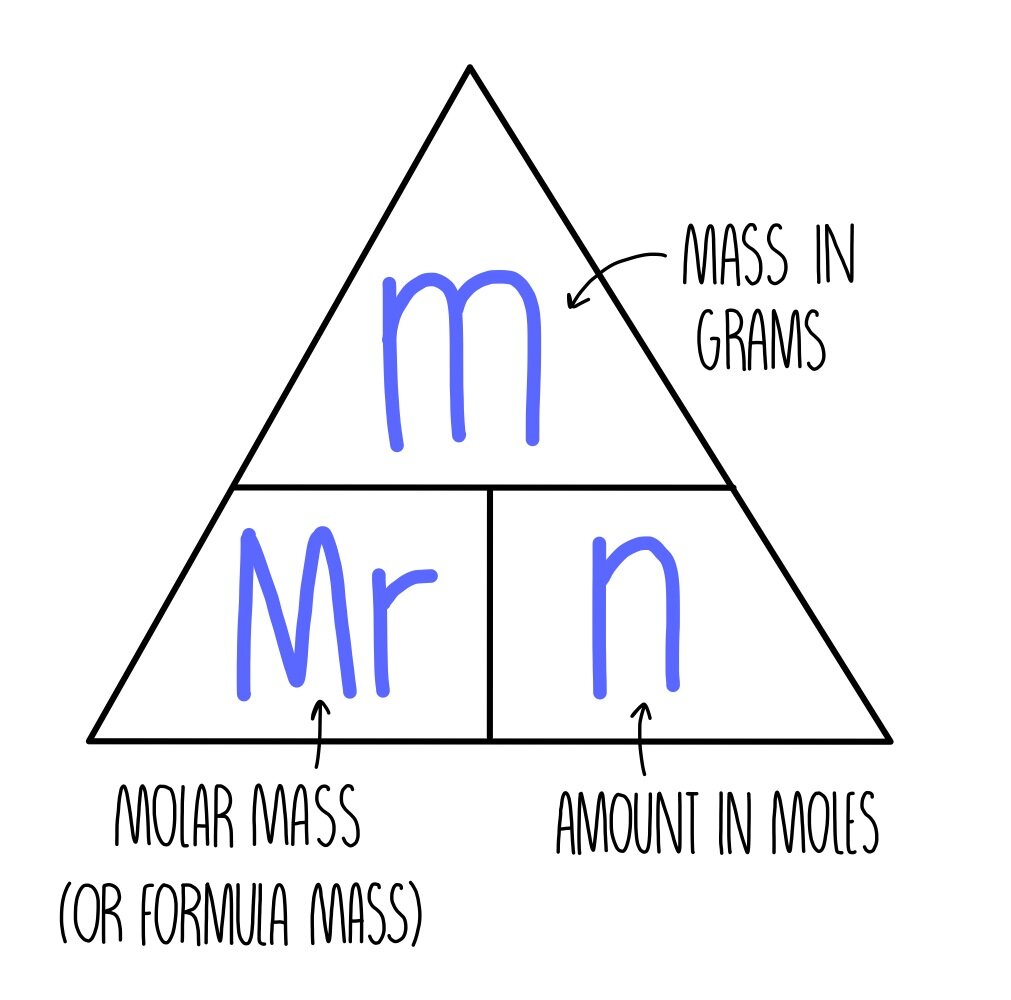
Chemical formulae, equations and calculations GCSE — the science sauce
As given to you, the molecular mass, M r, is most likely the relative molecular mass, sometimes called molecular weight, which is defined as molecular mass divided by 1 12th of the mass of a single, unbound carbon-12 atom. relative molecular mass = molecular mass 1 u For water, the relative molecular mass is M r = 18.015u 1u = 18.015
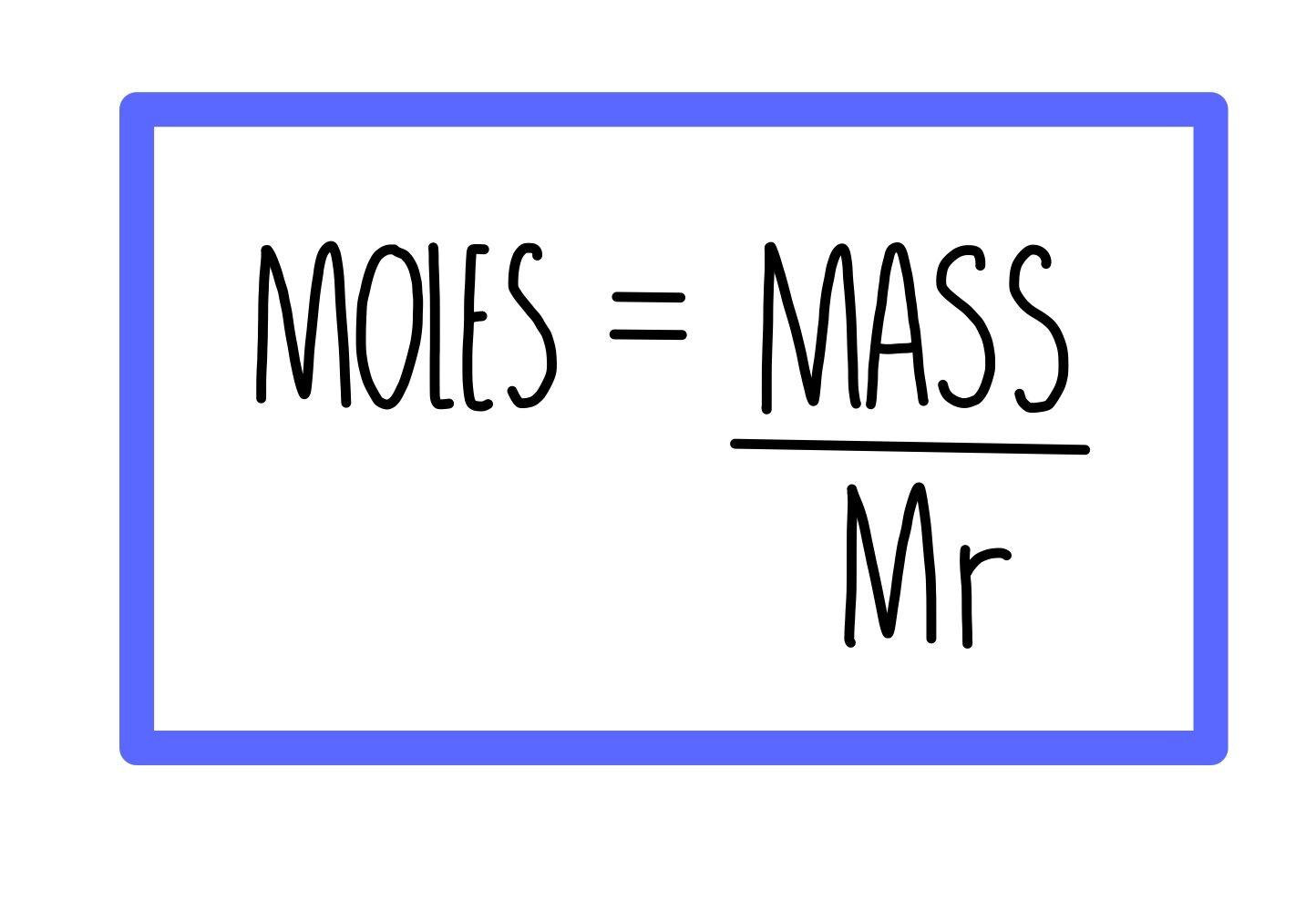
Chemical formulae, equations and calculations GCSE — the science sauce
Moles and masses , and relative formula mass are closely related. \ (moles = \frac {mass~ (g)} {M_r}\) You can imagine these properties of a substance in a triangle. You can reconfigure the.
CBATuition
David Park. 4 years ago. First, you can calculate the molar mass of FeCl2 by adding the molar masses of Fe (55.845 g/mol) and 2 atoms of Cl (2 times (35.446 g/mol). This gives a molar mass of 126.737 g/mol. Since each mole is 126.737 grams, you multiply 3.5 mols by 126.737 grams, giving you 443.58 grams.